|
|
| Overarching Regulation | |
|---|---|
| Regulations concerning the Hygiene Supervision over Cosmetics | |
| Competent Authority | |
| China Food and Drug Administration (CFDA) | |
| Main Supporting Rules | |
| 1 Jul 2007 | Hygienic Standard for Cosmetics |
| 1 Apr 2010 | Requirements for Application and Acceptance of Administrative Licensing for Cosmetics |
| 1 Jul 2011 | Guidance on Application and Review of New Cosmetic Ingredients |
| 1 Oct 2009 | GB 5296.3-2008 General Labeling for Cosmetics |
China’s current cosmetic regulatory system is founded on the overarching “Regulations concerning the Hygiene Supervision over Cosmetics (1989)”, supported by a series of subsidiary rules, standards and guidance documents issued by the former regulator, the Ministry of Health (MOH) and the current competent authority, China Food and Drug Administration (CFDA).
In the last two decades, China’s cosmetics industry has undergone tremendous change. Currently, the Chinese cosmetic market ranks as the third largest globally and is also the worlds’ emerging market, exhibiting the greatest average annual growth rate. To make the cosmetic regulatory framework consistent with the current industry situation, in the second half of 2013, CFDA launched a far reaching campaign set on thoroughly amending the overarching cosmetics regulation. All stakeholders will be significantly affected by the revised regulation. The amendment is not simply a refinement of its predecessor but represents a complete overhaul, which will see the definition, classification and registration requirements of cosmetics completely changed. Once effective, the new regulation will necessitate that, both domestic and overseas cosmetic companies adopt new compliance strategies.
Contents |
Part 1 Regulatory Framework and Competent Authority
Existing Main Cosmetic Regulations in China
|
Type |
Regulation |
Remark |
Effective Date |
Status |
|
|
Overarching |
Regulations concerning the Hygiene Supervision over Cosmetics 1989 《化妆品卫生监督条例》 |
Promulgated by MOH and acts as the overriding regulation for cosmetics in China |
1 Jan 1990 |
In force, but being revised |
|
|
Hygiene Standard |
For Product |
Hygienic Standard for Cosmetics 2007 《化妆品卫生规范》 |
A fundamental standard for cosmetic products and ingredient, containing prohibited and restricted substance lists and testing methods |
1 Jul 2007 |
In force, but being revised |
|
For Manufacturer |
Hygienic Standard for Production Enterprises of Cosmetics 2007 《化妆品生产企业卫生规范》 |
A GMP-like guidance for cosmetic manufacturers in China |
1 Jan 2008
|
In Force |
|
|
Licensing |
Requirements for Application and Acceptance of Administrative Licensing for Cosmetics 2009 《化妆品行政许可申报受理规定》 |
Specifies registration dossiers for imported cosmetics, domestic special use cosmetics and new cosmetic ingredient |
1 Apr 2010
|
In Force |
|
| Administrative Measures on Filing of Domestic Non-Special Use Cosmetics 《国产非特殊用途化妆品备案管理办法》 | Guidance for filing of domestic non-special use cosmetics |
1 Oct 2011 |
In Force | ||
| Requirements for Fling of Domestic Non-special Use Cosmetic Products 《国产非特殊用途化妆品备案要求》 | New Guidance for filing of domestic non-special use cosmetics | 30 Jun 2014 | Replaces the above measures since 30 Jun 2014 | ||
|
Guidance on Application and Review of New Cosmetic Ingredient 2011《化妆品新原料申报与审评指南》 |
Formulated especially for registration of new cosmetic ingredients |
1 Jul 2011 |
In Force |
||
|
Guidance on Application and Review of Children's Cosmetics 2012 《儿童化妆品申报与审评指南》 |
Formulated especially for registration of children’s cosmetics |
1 Feb 2013 |
In Force |
||
|
Testing |
Requirements for Cosmetic Administrative Licensing Testing 2010 《化妆品行政许可检验规范》 |
Sets out Testing requirements for imported cosmetics, domestic special use cosmetics and new cosmetic ingredient |
11 Feb 2010 |
In Force |
|
|
Labeling |
Provisions on the Administration of Cosmetics Labeling and instruction 《化妆品标签说明书管理规定》 |
Standardizes required info in label and instruction of cosmetic products |
/ |
Not Published yet |
|
|
GB 5296.3-2008 General Labeling for Cosmetics 《GB 5296.3-2008消费品使用说明化妆品通用标签》 |
Details required info on cosmetic labels |
1 Oct 2009 |
In Force |
||
|
Naming |
Guide to the Naming of Cosmetics 2010《化妆品命名规定》 |
Details requirements for naming |
5 Feb 2 010 |
In Force |
|
|
IECIC |
Inventory of Existing Cosmetic Ingredients in China 2014 《已使用化妆品原料名称目录》 |
Ingredients included in it need not registration |
/ |
IECIC 2014 is a draft |
|
|
Import & Export |
Administrative Measures on Inspection and Quarantine of Import and Export Cosmetics 《进出口化妆品检验检验监督管理办法》 |
Regulating the import and export of cosmetics |
1 Feb 2012 |
In Force |
|
Competent Authority of Cosmetics
1. China Food and Drug Administration (CFDA) was established on the basis of the former State Food and Drug Administration (SFDA), rebranded and elevated to a ministerial-level agency following a governmental restructuring in March 2014. CFDA is created to largely reduce the overlap of supervisory power shared by several departments and streamline regulation of food, drug and cosmetics safety.
In term of cosmetics, CFDA is in charge of comprehensive supervision on cosmetics, including domestic cosmetics production and registration of cosmetic products (special use cosmetics and imported non-special use cosmetics) and new ingredients, under which there are three sub-departments related to cosmetics:
- The Administrative Service Center is responsible for receiving cosmetics application cases and conducting format check on the dossiers submitted.
- The Dept. of Drug and Cosmetics Registration manages cosmetics administrative licensing and formulates cosmetics-related regulations.
- The Dept. of Drug and Cosmetics Supervision’s main duty is to oversee the production and operation activities of cosmetics nationwide and monitoring cosmetic undesirable effects.
2. The Cosmetic Registration Review Center under CFDA focuses on the technical review of cosmetic products and new cosmetic ingredients applying for registration. There is a technical Review Committee for cosmetics, mainly consisting of four distinct expert panels:
- Expert group to review product name/label
- Expert group to review product formula
- Expert group to review micro and chemical data
- Expert group to review toxicology data
3. The Food and Drug Administrations at provincial level under CFDA are in charge of registration of domestic non-special use cosmetics and issuance of cosmetics manufacturers’ production license, namely the Hygiene License for Production Enterprises of Cosmetics.
4. The General Administration of Quality Supervision, Inspection and Quarantine (AQSIQ)’ main supervisory scope is the import and export inspection of cosmetics.
5. The State Administration for Industry & Commerce (SAIC) manages cosmetic advertising, cosmetics trade mark registration and commercial activities.
Part 2 Cosmetic Products
Before being used, marketed or imported into China, cosmetic products must get approval from CFDA or provincial FDAs according to the product category.
Definition of Cosmetic Products
Cosmetic products refer to chemical products for daily use intended to be applied on any external part of human body (such as skin, hair, nails, lips etc.) by spreading, spraying or other similar ways to keep the body clean, eliminate unpleasant odor, protect skin, and improve appearance and beauty.
Cosmetics have various definitions in different countries (regions). How to determine whether a product falls in the scope of cosmetics in China? You may take into consideration the following three aspects:
|
Aspects |
Yes |
No |
|
Usage |
Smearing, spraying or other similar ways like rubbing |
Oral administration or injection |
|
Applied body parts |
Any external part of the human body, such as skin, hair, nails, lips |
Teeth or oral mucosa |
|
Functions and purposes of use |
Skin care, to make the body hygienic, to eliminate undesirable odors, to enhance the beauty of the appearance |
Prevent and treat diseases |
Classification of Cosmetic Products
Cosmetic products in China are divided into two categories.
|
non-special use cosmetics (non-SUC)
|
|
|
special use cosmetics (SUC) |
|
Whitening products have been re-classified as special use cosmetics and fall into the category of “freckle-removing” cosmetics.
Stakeholders should follow regulatory requirements for freckle-removing cosmetics to fulfill registration obligations which require extensive testing. For whitening products already available on the market, cosmetic companies are required to retrospectively fill any data gaps by submitting necessary testing data and other missing information. It is noteworthy that applicants of whitening products only providing physical over should register according to registration rules for imported non-special use cosmetics since they present relatively lower risks to consumers.
From 30 Jun 2015, China will ban the manufacture and import of whitening products without obtaining certificates for special use cosmetics.
Registration of Cosmetic Products
At present, imported cosmetics, domestic special use cosmetics and new cosmetic ingredients require pre-market registration with CFDA, while domestic non-special use cosmetics are subject to post-market filing with the provincial FDA. The registration of non-SUC is less stringent and demanding than that of SUC.
| Category | Obligation | Certificate | Department |
| Imported non-SUC | Pre-market filing | Filing certificate | CFDA |
| Imported SUC | Pre-market administrative licensing | Administrative license | CFDA |
| Domestic non-SUC | Post-market filing | Filing certificate | Provincial FDA |
| Domestic SUC | Pre-market administrative licensing | Administrative license | CFDA |
| New ingredient | Pre-market administrative licensing | SFDA’s approval announcement | CFDA |
Under new management system, from 30 Jun 2014, cosmetics manufacturers no longer need to obtain filing certificates, but instead need only to notify provincial FDA through an online platform of the product formula and sales packaging (including labels and instructions for use) prior to marketing. Applicants are required to record the product information, including formula, packaging, description of production process, product technical requirements and testing reports for future examination. In order to assist producers in China, CFDA has prepared a guidance document, “Requirements for Filing of Domestic Non-special Use Cosmetic Products”, which details all information required and the filing procedures.
The new system is much like the management of cosmetics in the EU and will reduce pre-market requirements for domestic non-special use cosmetics and significantly enhance post market supervision. Animal testing will no longer be a mandatory requirement for registration of non-SUC produced in China.
Quite a few complaints have been made by foreign cosmetic companies and industrial associations about Chinese government’s biased treatment of domestically-made non-SUC in comparison to imported counterparts. For the same kind of products, imported ones are subject to pre-market registration with CFDA while domestic ones only need to obtain a filing certificate from the provincial FDA within two months after cosmetic products are placed on the market.
The situation is to be changed. CFDA will delegate regulatory supervision and approval of imported non-special use cosmetics to provincial FDAs in a devolutionary process, starting from the lowest risk product categories before gradually encompassing all non-special use products. Provincial FDAs that have already set up cosmetics technical review centers and possess a contingent of relevant experts can make an application for official CFDA accreditation to the CFDA and will be officially qualified to approve products after receiving professional training by CFDA.
Registration Procedure
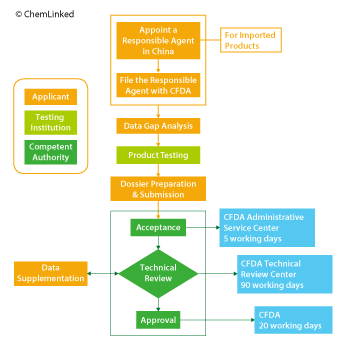
Step 1: Assign a Responsible Agent in China (imported products)
The pre-condition for registering imported cosmetics is to designate a Responsible Agent (RA), who must be a Chinese legal entity. The RA could be:
- A subsidiary based in China;
- Importer; or
- A third party, like REACH24H Consulting Group
Appointment of a capable Chinese responsible agent is a hugely important step. The selected intermediate can timely communicate with CFDA technical review experts and make quick responses to any questions raised and keep confidential key product information, such as production process and formula. A power of attorney needs to be signed and filed with CFDA.
Step 2: Check the compliance of formula, label and claim
It is not allowed to contain any prohibited substances or use restricted substances beyond permitted concentrations and applications scopes outlined in the Hygienic Standard for Cosmetics 2007. Meanwhile, it is also necessary to check if there is any new ingredient contained in the formula because new cosmetic ingredients also need CFDA’s approval.
Step 3: Arrange Product Testing
Cosmetic samples must be tested by CFDA-approved labs in China even if they have been tested and assessed as “safe” abroad. Testing items and duration depend on the type and property of cosmetic products.
Step 4: Submit Dossier, Technical Review and Approve
Applicants should submit registration documents and product samples to CFDA. Generally speaking, it takes 5 days for CFDA’s Administrative Service Center to perform a format check on all submitted documents and issue an acceptance notice, 90 days for the Registration Review Center to conduct the technical review of the product and 20 days for the CFDA to issue an approval certificate.
There are four possible results of technical review:
- Approval
- Approval subject to amendment of submitted dossier (if product Chinese name modifies or the description, format and writing of submitted documents need to be further improved )
- Additional information requested (if contents of dossier need to be revised; additional testing data or a verification test is needed; etc.)
- Rejection (if the dossier contents or product samples are false; the formula or testing result shows safety problem; etc.)
The CFDA expert panel will extensively review the following aspects:
- Product label and name: the applicant should concentrate on mandatory labeling items, warning statement, authentic Chinese translation of imported product label, appropriate efficacy claims and the correct SPF identification.
- Product formula: the use of prohibited ingredients listed in Hygienic Standard for Cosmetic 2007 is not permitted; cosmetics for increasing hair growth, slimming and breast beautification need a full description of efficacy ingredients and explanation and rationale for their usage, in addition to information of production process and quality specification.
- Hygienic chemical and microbiological testing data: all testing results analyzing the presence of heavy metals, microorganism, restricted and active ingredients must meet relevant requirements and confirm with formula statement.
- Toxicological data: the product should not cause obvious skin and eye irritation and elicit no obvious skin allergic reaction and /or photo toxicity; Infant or children products will be subjected to higher safety standards.
- Human safety evaluation: animal testing is required prior to human trials; human trials should demonstrate no obvious adverse reactions of cosmetics to human body.
Registration Duration and Cost
The duration and cost of testing and the whole registration process depend on the category and property of cosmetic products.
In generally it takes 40-60 days for imported non-SUC to be tested and 4-5 months to be registered. It takes 80 days for SUC to be tested and 6 months to be registered. Sunscreens and products for hair growth, body shaping and breast enhancement need more time since additional efficacy tests are required.
| Product | Testing Time | Whole Cycle |
| Imported non-special use | 40-60 days | 4-5 months |
| Special use | 80 days | 6 months |
| special use cosmetics for hair growth, slimming, breast beauty or sun protection (need efficacy additional test) | 150 days | Dependable |
The registration cost consists of testing fee, translation fee, notary fee and consulting fee (if a regulatory consultant is involved). CFDA does not charge any administration fee for registration.
Registration Dossier
The table below shows required documents for registration of imported cosmetic and domestic special use cosmetics:
|
Registration Documents |
Imported non-SUC |
Imported SUC |
Domestic SUC |
|
Application form |
√ |
√ |
√ |
|
Chinese product naming statement |
√ |
√ |
√ |
|
Product formula |
√ |
√ |
|
|
Description and diagram of the production process |
|
√ |
|
|
Quality control specification |
√ |
√ |
√ |
|
Original packaging (including label and instructions for use) |
√ |
√ |
√ |
|
Testing reports |
√ |
√ |
√ |
|
Safety assessment report (for risk-concern substance) |
√ |
√ |
√ |
|
Scientific reference of efficacy ingredients and their use for hair growth, slimming and breast beautification cosmetic products |
|
√ |
√ |
|
Power of Attorney and business license of the CFDA-recorded responsible person in China |
√ |
√ |
|
|
Letter of commitment that cosmetic ingredients meet the requirements for prohibited or restricted high-risk substances from Mad Disease Area |
√ |
√ |
|
|
Documents to prove manufacture and sale of the product in the country/region of origin |
√ |
√ |
|
|
Other documents that are helpful and necessary for review |
√ |
√ |
|
|
Product Technical Specification |
√ |
√ |
√ |
|
Examination and verification opinions on the production hygiene condition from the provincial FDA |
|
|
√ |
|
One sample |
√ |
√ |
√ |
Testing
Only testing reports issued by testing institutions approved by CFDA are valid for registration of cosmetic products and new ingredients.
Testing items depend on the type of cosmetic products. Generally, they are divided into microbiological, hygienic chemical, toxicological testing and human safety items.
Table 1. Microbiological Testing Items
|
Testing items |
Non-special use cosmetics |
Special use cosmetics |
||||||||
|
Hair growth |
Hair dye |
Hair perming |
Depilating |
Breast beauty |
Slimming |
deodorant |
Freckle removing |
Sunscreen |
||
|
Aerobic Bacterial Count |
√ |
√ |
|
|
|
√ |
√ |
|
√ |
√ |
|
Fecal Coliforms |
√ |
√ |
|
|
|
√ |
√ |
|
√ |
√ |
|
Staphylococcus Aureus |
√ |
√ |
|
|
|
√ |
√ |
|
√ |
√ |
|
Pseudomonas Aeruginosa |
√ |
√ |
|
|
|
√ |
√ |
|
√ |
√ |
|
Molds and Yeast Count |
√ |
√ |
|
|
|
√ |
√ |
|
√ |
√ |
Note:
- Nail polish removers and products with ethanol ≥ 75% (w/w) do not require microbiological tests.
- Products without ingredients to inhibit microorganism (such as physical depilating products and pure botanical hair dyes) need microbiological tests.
- If two or more independent small packages or delimited products (such as pressed powder, eye shadow and blush) in a sample package, they share the same product name but have different ingredients, each product contained needs microbiological tests.
Table 2. Hygienic Chemical Testing Items
|
Testing items |
Non-special use cosmetics |
Special use cosmetics |
||||||||
|
Hair growth |
Hair dye |
Hair perming |
Depilating |
Breast beauty |
Slimming |
Deodorant |
Freckle removing |
Sunscreen |
||
|
Mercury |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
|
Lead |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
|
Arsenic |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
|
Methanol |
|
|
|
|
|
|
|
|
|
|
|
Formaldehyde |
|
|
|
|
|
|
|
√ |
|
|
|
Thioglycollic acid |
|
|
|
√ |
√ |
|
|
|
|
|
|
Hydroquinone, Phenol |
|
|
|
|
|
|
|
|
√ |
|
|
Sexual Hormones |
|
√ |
|
|
|
√ |
√ |
|
|
|
|
UV filters |
|
|
|
|
|
|
|
|
|
√ |
|
Oxidative Hair Dyes |
|
|
√ |
|
|
|
|
|
|
|
|
Cantharidin,Chlormethine |
|
√ |
|
|
|
|
|
|
|
|
|
PH |
|
|
|
√ |
√ |
|
|
|
√ |
|
|
α-Hydroxy Acid |
|
|
|
|
|
|
|
|
|
|
|
Antibiotics and Metronidazole |
|
|
|
|
|
|
|
|
|
|
|
Antidangdruff agents |
|
|
|
|
|
|
|
|
|
|
Note:
- Products with total content of ethanol and isopropanol ≥ 10 % (w/w) require a methanol test.
- In addition to sun protection cosmetics, products with UV filter (excluding titanium dioxide and zinc oxide) ≥ 0.5 % (w/w) also need UV filter test.
- Products that contain α-Hydroxy Acid concentration ≥ 3 % (w/w) need α-Hydroxy Acid and PH tests.
- Products claiming acne treatment require Antibiotics and Metronidazole tests.
Table 3 Toxicological testing items for non-SUC
|
Testing Items |
Hair care |
Skin care |
Make-up |
Nail care |
fragrance |
|||
|
Products that are liable to contact with eyes |
General skin care products |
Products that are liable to contact with eyes |
General make-up |
Eye-applicable make-up |
Products for lip care and applied on lips |
|
|
|
|
Acute Dermal Irritation Test |
√ |
|
|
|
|
|
√ |
√ |
|
Acute Eye Irritation Test |
√ |
|
√ |
|
√ |
|
|
|
|
Repeated Dermal Irritation Test |
|
√ |
√ |
√ |
√ |
√ |
|
|
Note:
- Products with UV filter (excluding titanium dioxide and zinc oxide) ≥ 0.5 % (w/w) also need Skin Phototoxicity Test and skin sensitisation test.
- Body washing products, facial masks and facial cleansers need not repeated dermal irritation test.
Table 4 Toxicological testing items for SUC
|
Testing items |
Special use cosmetics |
||||||||
|
Hair growth |
Hair dye |
Hair perming |
Depilating |
Breast beauty |
Slimming |
Deodorant |
Freckle removing |
Sunscreen |
|
|
Acute Eye Irritation Test |
√ |
√ |
√ |
|
|
|
|
|
|
|
Acute Dermal Irritation Test |
|
|
√ |
|
|
|
|
|
|
|
Repeated Dermal Irritation Test |
√ |
|
|
|
√ |
√ |
√ |
√ |
√ |
|
Skin Sensitisation Test |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
|
Skin Phototoxicity Test |
√ |
|
|
|
|
|
|
√ |
√ |
|
Salmonella Typhimurium/Reverse Mutation Assay/ Gene Mutation |
√ |
√ |
|
|
√ |
√ |
|
|
|
|
In Vitro Mammalian Cells Chromosome Aberration Test |
√ |
√ |
|
|
√ |
√ |
|
|
|
Table 5 Human safety testing items for SUC
|
Testing items |
Hair Growth |
Depilating |
Breast beauty |
Slimming |
Deodorant |
Freckle-removing |
Sunscreen |
|
Skin Patch Test |
|
|
|
|
√ |
√ |
√ |
|
Human Trial |
√ |
√ |
√ |
√ |
|
|
|
CFDA-Approved Testing Institutions
So far there are 27 testing labs approved by CFDA to undertake the testing of imported cosmetic products, domestic special use cosmetics and new cosmetic ingredients. They are divided into two types due to different testing items.
1) The Hygiene safety testing institutions perform all microbiological, hygienic chemical and toxicological tests. (These tests should be performed by the same testing agency).
2) The Human safety testing institutions conduct human safety tests (including human skin patch test and human trials) and cosmetic sunscreens efficacy test. It is noted that human safety tests should be conducted in a single testing institute.
CFDA-accredited 27 testing agencies for special use cosmetics, imported cosmetics and new cosmetic ingredients
|
Type |
Testing institution |
|
Hygiene safety Testing institution (microbiological test, hygienic chemical test, toxicological test) |
Institute of Environmental Health and Related Product Safety, China CDC |
|
Human safety testing institutions (human safety test and test in vivo of UV protection efficacy of cosmetic sunscreens) |
General Hospital of Chinese People's Liberation Army Air Force |
Labeling of Cosmetic Products
Cosmetics labels are subject to review by CFDA expert panel during registration and also checked by CIQs when products arrive at port. Many exporters lack adequate knowledge about correct Chinese compliant labeling for their cosmetics, demonstrated by high volume of cosmetic products destroyed or rejected at port by CIQs due to substandard labels.
According to Instruction for Use of Consumer Products—General Labelling for Cosmetics (GB 5296.3-2008), the following information shall be indicated on labels of the imported cosmetics:
- Product name (in line with Requirements on Naming of Cosmetics)
- Name and address of manufacturer, distributor
- Country of origin
- Approval ID number (the ID number on the administrative license or record-filing certificate, such as “国妆备进字J20130000”)
- List of ingredient (all ingredients with concentration over 1.0% must be indicated with Chinese INCI names and listed in descending order of “quantity”; in absence of INCI name, the corresponding name in Chinese Pharmacopoeia, its chemical or botanical name can also be adopted)
- Net content
- Production date and shelf life or the batch number and expiration date
- Warnings (for products containing restricted substance, e.g. Zinc phenolsulfonate, the term “avoid to contact with eyes” shall be indicated on the label; and special use cosmetics e.g. hair dye)
- Instruction and storage condition (provided if inadequate storage impacts safety)
- Other info (the local distributor or wholesaler can be indicated as well)
Two options are available for labeling the imported cosmetics. Companies can design the label especially for the Chinese market according to Chinese labeling regulations or use the original package with a China compliant over-label.
Claim of Cosmetic Products
Cosmetic companies cannot claim functions or features that the products do not possess. Medicinal or therapeutic function claims and any misleading wordings are prohibited. Local expertise will be necessary for balancing compliance with Chinese claim requirements and the addressing the interests of manufacturers who rely on the added value derived from inclusion of certain claims in their marketing strategies. Claims on some imported products, such as “hypoallergenic”, “dermatologically tested” or “100% natural”, would be challenged by CFDA’s expert panel.
CIQ Inspection of Imported Cosmetics
After the products are approved by CFDA, foreign firms can start exporting cosmetic products to China immediately. Distributors or importers in China need to apply for CIQ inspection by submitting required documents, such as CFDA product approval license or certificate, product formula, Chinese label samples, etc. The inspection process includes on-site examination of the label and package, sampling and testing and the issuance of the Certificate of Inspection and Quarantine of Imported Goods, which is the ultimate requirement for custom clearance.
Production Licensing of Cosmetics in China
The former supervisory system placed cosmetics production under the dual regulation of both CFDA and AQSIQ. A cosmetics manufacturer in China was required to not only obtain a Hygiene License for Production Enterprises of Cosmetics from provincial FDAs but also a Cosmetics Production License from provincial quality and technology supervision administration under AQSIQ. The redundancy inherent in the old model whereby two departments issued two similar licenses decreased the efficiency of cosmetics supervision and also overburdened the cosmetic industry. To reduce regulatory overlap and enhance working efficiency, CFDA integrated the two licenses to streamline the cosmetic production licensing system in China in 2014.
At present, newly established cosmetics manufacturers in China have one less regulatory authority to deal with and just need to obtain the Hygiene License for Production Enterprises of Cosmetics from provincial FDAs.
Part 3 Cosmetic Ingredient
Cosmetic Ingredient Lists
China divides cosmetic ingredients into:
- New cosmetic ingredient; (need registration with CFDA)
- Existing cosmetic ingredient;
|
Type |
List Name |
Current Version (year) |
Number Contained |
||
|
Existing |
Inventory of Existing Cosmetic Ingredients in China (IECIC) |
2014 (Draft) |
8641 |
||
|
Restricted substances in cosmetic products |
List of Substances Restricted in Cosmetic Products |
2007 |
73 |
||
|
List of Preservatives Permitted in Cosmetic Products |
2007 |
56 |
|||
|
List of UV Filters Permitted in Cosmetic Products |
2007 |
28 |
|||
|
List of Colorants Permitted in Cosmetic Products |
2007 |
156 |
|||
|
List of Hair Dyes Permitted in Cosmetic Products |
2007 |
93 |
|||
|
New |
List of Approved New Cosmetic Ingredients |
2012 |
9 |
||
|
Prohibited |
List of Substances Prohibited in Cosmetic Products |
2007 |
1286 |
||
|
INCI Chinese Version |
List of Standardized Chinese INCI Name |
2010 |
15649 |
||
Inventory of Existing Cosmetic Ingredients in China (IECIC)
Ingredients listed in the IECIC are regarded as “existing” substances, which need not approval by CFDA prior to use in cosmetic products.
The issue of IECIC can be traced back to 2003, since when the government started to regulate the allowable cosmetic ingredients through the positive list. On 27 April 2003, the Ministry of Health (MOH) released the IECIC 2003, which is an internally circulated list among related departments. A large number of recognized and globally accepted ingredients that have been scientifically established safety and have a long history of safe usage are not included in the IECIC, which creates a major issue for multinational companies.
From 1 Sep 2008, CFDA took over the full responsibility of cosmetic supervision from the MOH. With the rapid development of cosmetic industry and soar in import and export trade, in order to facilitate the registration of new cosmetic ingredients, CFDA started to improve the existing ingredient inventory. In 2013, CFDA published 2 batches containing 2085 substances based on three drafts released in 2012.
In an effort to improve the situation, in 2012, the CFDA drafted three batches of existing cosmetic ingredients, totaling 3,667 ingredients.
In 2013, CFDA just finalized the first two batches, leaving the number of ingredients in the third batch a mistery.


The third batch was expected to be finalized. However, on 21 Jan 2014, a consolidated list of IECIC 2014 (draft) containing 8641 existing cosmetic ingredients was released by CFDA for public comments, which indicates CFDA has chosen to abandon a batch-wise approach and instead published the integrated existing cosmetics inventory. The release of this consolidated list is a favorable turn in the process of cosmetic ingredient regulation in China. The scope of used ingredients in China will become much more specific and transparent than before.
In case that an ingredient in the IECIC is also a restricted substance in the Hygienic Standard for Cosmetics 2007, it should be used in conformity with corresponding restriction requirements, such as application scope, maximum permitted concentration in the finished cosmetic products, warnings, etc. Please click here to download the English version of IECIC 2014 (draft) prepared by ChemLinked.
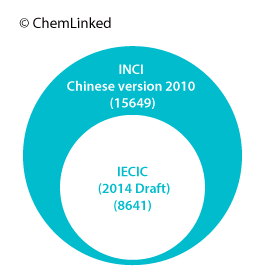
Cosmetic companies might confuse IECIC with the INCI Chinese Version and falsely regard ingredients in the latter as “used or existing” in China. It is worth clarifying that the INCI Chinese Version is translated with the main purpose to standardize the translation of INCI name and cosmetic labeling and instructions, which is certainly not a criterion for determining the regulatory status of an ingredient.
The INCI Chinese Version was firstly published by the MOH in 2007 with 12072 ingredients, which was translated according to the International Nomenclature Cosmetic Ingredient (INCI) names included in the International Cosmetic Ingredient Dictionary & Handbook Tenth Edition (2004) (ICI Dictionary) managed by the Personal Care Products Council (PCPC), previously the Cosmetic, Toiletry and Fragrance Association (CTFA). The List is updated accordingly with the expansion of the ICI Dictionary.
The latest version containing 15649 ingredients was translated and released by CFDA in 2010, based on its twelfth Edition (2008). Cosmetic manufacturers are required to label the ingredients using the standard Chinese names if they are covered by the List.
According to the PCPC, the ICI Dictionary has been updated to its 14th edition in 2012 with more than 19,000 INCI labeling names for the United States, the European Union and other countries. These are cross-referenced to more than 66,000 trade and technical names and nearly 4,300 suppliers from 106 countries. The total number of ingredients of IECIC 2014 is just half of that in the ICI Dictionary so a great many substances that are almost internationally accepted to be used in cosmetic products and personal care products still cannot be used in China without approval.
List of Approved New Cosmetic Ingredients
During 2004 to 2012, there were only 9 new cosmetic ingredients that got approval from MOH and CFDA, indicating the extreme difficulty in registration of a NCI in China. From 2008 to 2012, 4 years has witnessed a meagre 3 NCIs successfully approved by CFDA in spite of the continued submission of application dossiers. According to statistics from CFDA, more than 130 dossiers have been submitted for the administrative licensing of NCIs.
The List of 9 Approved New Cosmetic Ingredients during 2004-2012
|
INCI Name |
Function |
Applicant |
Approved Year |
|
Alkyl (C12-C22) trimethyl ammonium, bromide and chloride |
Non-preservative |
Not disclosed |
Jun 2004 (MoH) |
|
Potassium Methoxysalicylate |
Brightener |
Shisheido |
Apr 2007 (MoH) |
|
Methylisothiazolinone |
Preservative |
Rohm Haas |
May 2007 (MoH) |
|
Carnitine Tartrate |
Active ingredient |
Not disclosed |
Jun 2008 (MoH) |
|
Lathyrus odoratus flower extract |
Skin conditioning |
Not disclosed |
Aug 2008 (MoH) |
|
Fructooligosaccharides |
Humectant |
Not disclosed |
Aug 2008 (MoH) |
|
Dimethoxytolyl Propylresorcinol |
Skin conditioning |
Unigen |
Mar 2012 (CFDA) |
|
Polymethacryloyl Lysine |
Humectant |
Not disclosed |
Mar 2012 (CFDA) |
|
Phenylethyl Resorcinol |
Brightener |
Symrise |
Dec 2012 (CFDA) |
|
Elaeagnus mollis oil |
Humectant & Antioxidant | Qierkang (A Chinese company) | Jun 2013 (draft) |
Lists of Prohibited and Restricted Ingredients
China regulates substances banned and restricted in cosmetic products in a way similar to in EU Cosmetic regulation, which includes lists of prohibited and restricted substances, colorants, preservatives, and UV filters. In China, these kinds of lists are contained in the Hygienic Standard for Cosmetics 2007, the standard that all cosmetic products and ingredients must adhere to, which also contains methods of toxicological test, hygienic chemical test and microbiological test along with efficacy evaluation methods.
Substances that draw the most attention of the CFDA review experts are usually substances banned from use in cosmetic products, such as Dioxane, Phenol, Acrylamide, Asbestos, Nitrosamines, etc.
If some prohibited substances are present in cosmetics as by products or impurities, which during the prodcution process cannot be technically avoided, an exception is made providing that the finished products meet the general requirements outlined in the Hygienic Standard for Cosmetics 2007. Namely, the finished products may not harm human health under normal, reasonable and forseeable conditions of use. For instance, Dioxane is banned but it is currently technically impossible to prevent residual carryover, so CFDA set a maximum limit of 30mg/kg, which is deemed toxicologically acceptable.
The Hygienic Standard for Cosmetics 2007 also prescribes limits for heavy metals and methanol in cosmetic products:
|
Toxic Substance |
limit (mg/kg) |
Revised Limit (mg/kg) |
|
Mercury |
1 |
1 |
|
Lead |
40 |
10 |
|
Arsenic |
10 |
4 |
|
Methanol |
2000 |
2000 |
CFDA is amending the Hygienic Standard for Cosmetics 2007 and renames it as “Technical Safety Standard for Cosmetics” (CL news on 7 Dec 2012). The new standard is divided into two main parts, the body text with general requirements for safe use of cosmetic ingredients and products and three annexes containing lists of prohibited and restricted substances and test methods.
On 30 Dec 2012, the body text was released by CFDA for public comments. It greatly reduces the limits for the lead and the arsenic in cosmetic products, from 40 mg/kg and 10 mg/kg to 10 mg/kg and 4 mg/kg, respectively. The lists of prohibited and restricted subtances would also be modified.
Definition of New Cosmetic Ingredient (NCI)
According to the article 9 of the overarching cosmetic regulation, a new cosmetic ingredient is a natural or artificial ingredient that is applied in cosmetic products for the first time in China. Before being used to make cosmetics, it must be approved by CFDA. Basically, an ingredient is deemed as “new” if not included in IECIC.
In the case that a new ingredient is identified as a new chemical under the China chemical regulation, it should be notified to the Ministry of Environment (MEP) before manufacture or import [See Chempedia China NCSN (China REACH) for exhaustive compliance guidance].
In this instance, double registration under two different regulatory schemes is required prior to placing a cosmetic ingredient on the Chinese market.
Registration of New Cosmetic Ingredients
The NCI registration procedure is similar to that of cosmetic products but more costly and time-consuming. Applicants can refer to the Guidance on Application and Review of New Cosmetic Raw Materials to fulfill registration, which details the registration dossier, required toxicological tests and testing exemptions.
In the past once CFDA announced the approval of a NCI, all subsequent companies wishing to utilize the substance were permitted. The old policy has been complained as unfair to the applicant who has paid extensive efforts to gain the permit, which will ultimately hurt companies' initiative to do registration.
Under the new management scheme, cosmetic companies who want to utilize the same new ingredient are subject to separate registration. CFDA will no longer release online announcements to approve new cosmetic ingredients but issue “new cosmetic ingredient trial use certificate” for the applicant only. The period of validity is four years, during which applicants can manufacture, sell and use this new ingredient. If no adverse events are observed during the 4 years, the ingredient will be listed into IECIC allowing all cosmetics companies to freely utilize the substance. That is to say the applicant can enjoy the exclusive protection period of 4 years (CL news on 26 Jan 2014).
Registration Procedure
The registration procedure of new cosmetic ingredients is similar to that of special-use cosmetics shown in Figure 5.
Registration Dossier
- New cosmetic ingredient administrative license application form
-
Research & Development Report;
- R & D background, processes and other related technical information.
- The name and source of the ingredient, relative molecular weight, molecular formula, chemical structure and physical-chemical properties.
- The intended use and application scope of the ingredient in cosmetics, safe use limit, warning statement, etc.
- Basic description and diagram of the production process;
- The quality and safety control requirements of the ingredients, including specifications, quantitative/qualitative analytical methods, impurities and their control measures;
-
Toxicological safety evaluation material, including
- A summary of evaluation
- Toxicological testing data
- Safety evaluation data on potential risk substances (i.e., prohibited substances)
- The Power of Attorney and the Business License of the SFDA-recorded responsible agent by the overseas applicant;
- Testing sample verification reports (to demonstrate the consistency or to prove the new ingredient was employed in the actual toxicological tests);
- Analytical method validation reports (if the analytical method for identification and quality control was developed by the applicant itself, not derived from CFDA-published testing guidelines, the validation should be carried out by three separate CFDA-approved testing institutions)
- Other supportive documents
The toxicological safety assessment mainly focuses on identifying prohibited substances or toxic impurities present in cosmetic ingredients. For example, phenol is a common impurity in phenoxyethnol and a testing report of phenol would normally be provided either for phenoxyethanol itself or a finished cosmetic product containing this preservative. Dioxane, methanol, acrylamide and N-nitrosodiethanolamine are normally tested for certain ingredients and their safe concentration should be calculated to demonstrate their safety if trace levels are present in the finished formulation. Impurities that pose potential risks and toxicological evaluation data will be extensively reviewed by CFDA expert panel.
Toxicological Test
Applicants need to entrust a CFDA-accredited lab to prepare toxicological data demonstrated table below. The table below is a sample from Shanghai CDC, showing required testing items, duration, sample quantity and testing fee for a NCI:
|
No. |
Testing Item |
Testing Fee (RMB) |
Duration (Month) |
Sample (g/ml) |
|
|
1 |
急性经口毒性试验 |
Acute Oral Toxicity Test |
5000 (small rat) 8000 (big rat) |
2 |
100-200 |
|
2 |
急性经皮毒性试验 |
Acute Dermal Toxicity Test |
8000 |
2 |
150 |
|
3 |
急性皮肤刺激性试验 |
Dermal Irritation/Corrosion Test |
6000 |
1.5 |
50 |
|
4 |
多次皮肤刺激性试验 |
Repeated Dermal Irritation Test |
10000 |
2.5 |
100 |
|
5 |
急性眼刺激性/腐蚀性试验 |
Acute Eye Irritation/Corrosion Test |
6000 |
2 |
50 |
|
6 |
皮肤变态反应试验 |
Skin Sensitisation Test |
10000 |
3 |
100 |
|
7 |
皮肤光毒性试验 |
Skin Phototoxicity Test |
6000 |
1.5 |
100 |
|
8 |
鼠伤寒沙门氏菌/回复突变试验 |
Salmonella typhimurium/reverse mutation assay |
8000 |
2 |
50 |
|
9 |
体外哺乳动物细胞染色体畸变试验 |
In Vitro Mammalian Cells Chromosome Aberration Test |
15000 |
3 |
50 |
|
10 |
体外哺乳动物细胞基因突变试验 |
In Vitro Mammalian Cell Gene Mutation Test |
17000 |
4 |
50 |
|
11 |
哺乳动物骨髓细胞染色体畸变试验 |
In Vivo Mammalian Bone Marrow Cell Chromosome Aberration Test |
10000 |
2.5 |
50 |
|
12 |
体内哺乳动物细胞微核试验/小鼠骨髓细胞为何试验 |
Mammalian Erythrocyte Micronucleus Test |
10000 |
2.5 |
100 |
|
13 |
睾丸生殖细胞染色体畸变试验 |
Testicle Cells Chromosome Aberration Test |
10000 |
3.5 |
100 |
|
14 |
亚慢性经口毒性试验/90天经口毒性试验 |
Subchronic Oral Toxicity Test |
170000 |
9 |
2000 |
|
15 |
亚慢性经皮毒性试验/90天精品毒性试验 |
Subchronic Dermal Toxicity Test |
180000 |
9 |
250 |
|
16 |
致畸试验 |
Teratogenicity Test |
100000 |
6.5 |
1000-2000 |
|
17 |
慢性毒性/致癌性结合试验 |
Combined Chronic Toxicity/Carcinogenicity Test |
1800000 |
36-48 |
5000-10000 |
|
Test |
Exemption Cases |
||||
|
1. The ingredient is not used as a preservative, sunscreen, colorant or hair dye; and needs not to be added to restricted substances list in Hygienic Standard for Cosmetics |
2. The ingredient has met criteria (1) and has been listed in the inventory of existing ingredients in overseas authoritative orgs for more than 4 years and is not found to be hazardous in relevant literature |
3. The ingredient is proven to have a history of safe use as food ingredient by government or authoritative orgs |
4. Polymer with average molecular weight above 1000 Daltons |
5. Risk assessment of the cosmetic ingredient has been carried out by overseas authoritative orgs and the conclusion is that the ingredient is safe to be used in cosmetics |
|
|
acute oral and acute dermal toxicity |
√ |
√ |
All data are exempt but the conclusion, report of safety evaluation and other supportive documents in overseas countries are required. |
||
|
skin and eye irritation/corrosion |
√ |
√ |
√ |
√ |
|
|
skin sensitisation |
√ |
√ |
√ |
||
|
skin phototoxicity and photosensitivity |
√ (for UV-filters) |
√ |
√ |
√ |
|
|
mutagenicity |
√ (include at least a gene mutation test and a chromosome aberration test) |
√ |
|||
|
sub-chronic oral or dermal toxicity |
√ |
√ |
|||
|
teratogenicity |
|||||
|
chronic toxicity/carcinogenicity |
|||||
|
toxicokinetics and dynamics |
|||||
In principle, it is mandatory for applicants to submit animal testing data. However, applicants could also provide the toxicological information utilizing read-across data, QSAR and available clinical or epidemiological data to prove the safety of the NCI. There is however a risk that this data will be inadequate and ultimately prolong registration as the CFDA expert panel may be unsatisfied with these kinds of data and require re-submission of animal testing data.
Part 4 Animal Testing Issue
China’s mandatory requirement of animal testing for cosmetics registration has been a long standing controversy that makes current Chinese cosmetics regulatory framework incompatible with that of EU and Israel where such testing is prohibited for both cosmetic products and ingredients. The different animal testing policies have made global trade between these regions difficult. Chinese companies cannot sell cosmetics in EU if they are marketed in China and the European companies that stick to “cruelty-free” status cannot enter the Chinese market.
Under the existing regulatory regime, cosmetic companies are obliged to assign CFDA-approved labs to perform animal tests on their cosmetic finished products or ingredients even if they have been assessed as “safe” and used or marketed in other countries. Testing results drawn from non-animal testing methods are not likely to be trusted and accepted by CFDA technical review experts. However, 2013 witnessed a turning point for animal testing policy in China.
The abolishment of the obligatory animal testing requirement for domestic non-special use cosmetics is the most significant cosmetics policy shift in 2013.Toxicological tests can be exempted as long as the ingredients and the finished products are proven to be safe after risk assessment according to Guide for Risk Assessment of Possible Substances with Safety Risks in Cosmetics [CFDA Circular No. 339 of 2010].
Although it is still uncertain whether the new change will be extended to imported products, it is nonetheless a major milestone for China on its way to modernization of its animal testing policy and alignment of its regulatory framework with the global cruelty free trends.
News
- 26 Jan 2014 China Makes Significant Amendment to New Cosmetic Ingredients Registration
- 23 Jan 2014 China Drafts a Consolidated List of 8641 Existing Cosmetic Ingredients (IECIC 2014)
- 23 Dec 2013 China Officially Accepts Domestic Non-special Use Cosmetics without Animal Testing
- 7 Nov 2013 Chinese Authority Softens Stance on Mandatory Cosmetics Animal Testing
- 7 Nov 2013 Skin-whitening Products are Newly-classified as Special-use Cosmetic in China
- 6 Oct 2013 China Streamlines Cosmetics Production Licensing
- 28 Sep 2013 China to Amend the Top Cosmetics Regulation
- 27 May 2013 Registration of Imported Non-special Use Cosmetic Products in China decentralized to Provincial FDA
- 20 May 2013 Practical Guide to the Registration of New Cosmetic Ingredients in China
- 24 Dec 2012 Strict-ever Limits for Pb/As in Cosmetics in China
- 7 Dec 2012 China SFDA Updates the Hygienic Standard for Cosmetics
Expert Articles & E-books
- Martin Hu, 26 Jul 2013, Cosmetic Labeling Requirements in China: Cross Regulatory Compliance
- Martin Hu, 15 May 2013, Children's Cosmetics: Obligations for Foreign Stakeholders in the Chinese Market
- Tommy Kong, 8 Jun 2013, Steps to Export Cosmetic Products to China
- Tommy Kong, 8 Jun 2013, Pre-market Approval of New Cosmetic Ingredients in China
- Tommy Kong, 8 Jun 2013, Introduction of the Lists of Regulated Cosmetic Ingredients in China
- Tommy Kong, 8 Jun 2013, Introduction of the Regulatory Requirements of Wet Wipes in China
- Echo Cao, 18 Oct 2012, New Cosmetic Ingredients Application Process: Interpretation and Advice
- Martin Hu, 29 Nov 2010, Ebook03: Guidance on Application and Review of New Cosmetic Raw Materials